Congo red has strong tinting strength, low price that it was used in meat dyeing. Amino acid ionic liquids (AAILs) containing amino acid cations or anions are synthesized from natural amino acids, which has the characteristics of low toxicity. Nowadays, Fe3O4@SiO2@AAIL composites has seemly not been reported for the separation/analysis of congo red. In this work, Fe3O4@SiO2@AAIL nanoparticles were used as magnetic solid phase adsorbents in combination with UV spectrophotometry for separation of congo red.
| Published in | World Journal of Applied Chemistry (Volume 9, Issue 1) |
| DOI | 10.11648/j.wjac.20240901.12 |
| Page(s) | 7-14 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2024. Published by Science Publishing Group |
Congo Red, Amino Acid Ionic Liquids (AAILs), Solid Phase Extraction, UV Spectrophotometry
2.1. Equipment and Reagents
2.2. Preparation of Fe3O4@SiO2 @AAIL
2.3. Extraction Procedure
2.4. Sample Preparation
3.1. Characterization of Fe3O4@SiO2@ AAIL
3.1.1. The Characterization of FTIR
3.1.2. The Characterization of SEM
3.1.3. Characterization by XRD
3.2. Adsorption Process
3.2.1. The Effect of pH
3.2.2. Effect of Amount of Adsorbent
3.2.3. The Effect of Temperature
3.2.4. The Effect of Extraction Time
3.2.5. Effect of Ionic Strength
3.3. Adsorption Capacity
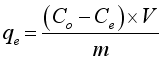
3.4. Elution Process
3.4.1. Eluent Selection
3.4.2. The Effect of Eluent Volume
3.4.3. The Effect of Elution Time
3.4.4. The Effect of Elution Temperature
3.5. The Repeated Times of Fe3O4@SiO2@AAIL
3.6. Interference Experiment
3.7. Analytical Performance
Sample | Added (g mL-1) | Found (g mL-1) | Recovery (%) | RSD intra-day (%) | RSD inter-day (%) |
|---|---|---|---|---|---|
City water | 0.00 | 0.77 | — | 2.8 | 3.0 |
0.51 | 1.25 | 97.1 | 3.8 | 7.9 | |
2.00 | 2.79 | 102.2 | 2.6 | 8.6 | |
3.00 | 3.72 | 98.6 | 2.9 | 4.9 | |
Beverage | 0.00 | 1.3 | — | 2.2 | 9.2 |
0.51 | 1.82 | 101.8 | 5.1 | 9.1 | |
2.00 | 3.27 | 98.3 | 4.5 | 7.4 | |
3.00 | 4.23 | 97.4 | 4.3 | 6.5 |
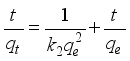
| [1] | Srilakshmi, C., & Saraf, R. (2015). Ag-doped hydroxyapatite as efficient adsorbent for removal of congo red dye from aqueous solution: synthesis, kinetic and equilibrium adsorption isotherm analysis. Microporous & Mesoporous Materials, 219, 134-144. |
| [2] | Sayğılı, G. A. (2015). Synthesis, characterization and adsorption properties of a novel biomagnetic composite for the removal of congo red from aqueous medium. Journal of Molecular Liquids, 211, 515-526. |
| [3] | Jin, L. N., Qian, X. Y., Wang, J. G., Aslan, H., & Dong, M. (2015). Mil-68 (in) nano-rods for the removal of congo red dye from aqueous solution. Journal of Colloid & Interface Science, 453, 270. |
| [4] | Li, X. S., Xu, L. D., Zhu, G. T., Yuan, B. F., & Feng, Y. Q. (2012). Zirconium arsenate-modified magnetic nanoparticles: preparation, characterization and application to the enrichment of phosphopeptides. Analyst, 137 (4), 959-967. |
| [5] | Chen, H., Deng, C., & Zhang, X. (2010). Synthesis of Fe3O4@SiO2@pmma core-shell-shell magnetic microspheres for highly efficient enrichment of peptides and proteins for maldi-tof ms analysis. Angewandte Chemie, 49 (3), 607–611. |
| [6] | Deng, M., Cheng, J., & Li, J. (2013). N-methylimidazolium modified magnetic particles as adsorbents for solid phase extraction of genomic deoxyribonucleic acid from genetically modified soybeans. Analytica Chimica Acta, 771 (7), 31-36. |
| [7] | Abolghasemi, M. M., Yousefi, V., & Piryaei, M. (2015). Double-charged ionic liquid-functionalized layered double hydroxide nanomaterial as a new fiber coating for solid-phase microextraction of phenols. Microchimica Acta, 182 (13-14), 2155-2164. |
| [8] | Guo, B., Ji, S., Zhang, F., Yang, B., Gu, J., & Liang, X. (2014). Preparation of c18-functionalized Fe3O4@SiO2 core-shell magnetic nanoparticles for extraction and determination of phthalic acid esters in chinese herb preparations. Journal of Pharmaceutical & Biomedical Analysis, 100 (21), 365-368. |
| [9] | Gong A, Zhu X. Dispersive solvent-free ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction coupled with HPLC for determination of ulipristal acetate [J]. Talanta, 2015, 131: 603. |
| [10] | Qin X, Zhu X. Determination of Allura Red in Food by Ionic Liquid ß-Cyclodextrin-Cross-Linked Polymer Solid Phase Extraction and High-Performance Liquid Chromatography [J]. Analytical Letters, 2016, 49 (2): 189-199. |
| [11] | H. M. Jiang, T. Yang, Y. H. Wang, H. Z. Lian, X. Hu. Magnetic soild-phase combined with graphite furnace atomic absorption spectrometry for speciation of Cr(III) and Cr(VI) in environment waters. Talanta. 2013 116: 361–367. |
| [12] | Li Q L, Wang L L, Wang X, et al. Magnetic metal-organic nanotubes: An adsorbent for magnetic solid-phase extraction of polychlorinated biphenyls from environmental and biological samples. Journal of Chromatography A, 2016, 1449: 39-47. |
| [13] | Wang P, Wang X, Yu S, et al. Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chemical Engineering Journal, 2016, 306: 280-288. |
| [14] | Li N, Chen J, Shi Y P. Magnetic reduced graphene oxide functionalized with β-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. Journal of Chromatography A, 2016, 1441: 24-33. |
| [15] | Qian W, Wang Y, Xu K, et al. Magnetic solid-phase extraction of protein by ionic liquid-coated Fe@graphene oxide. Talanta, 2016 160: 481-488. |
| [16] | Wu J, Zhao H, Xiao D, et aL. Mixed hemimicelles solid-phase extraction of cephalosporins in biological samples with ionic liquid-coated magnetic graphene oxide nanoparticles coupled with high-performance liquid chromatographic analysis. Journal of Chromatography A. 2016 1454: 1-8. |
| [17] | Wu Changzeng, Li Gongchun. Preparation and Application of Amino Acid Ionic Liquids [J]. Chemical Reagents, 2012 34: 933- 936. |
| [18] | Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. Journal of the American Chemical Society, 2011, 127: 2398-2399. |
| [19] | Huang R, Fang Z, Yan X, et al. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4, magnetic nanoparticles under neutral condition [J]. Chemical Engineering Journal, 2012, 197: 242-249. |
| [20] | Hong R Y, Zhang S Z, Han Y P, et al. Preparation, characterization and application of bilayer surfactant-stabilized ferrofluids [J]. Powder Technology, 2006, 170: 1-11. |
| [21] | Ma X, Wang J, Sun M, et al. Magnetic solid-phase extraction of neonicotinoid pesticides from pear and tomato samples using graphene grafted silica-coated Fe3O4 as the magnetic adsorbent [J]. Analytical Methods, 2013, 5:2809-2815. |
| [22] | Y. H. Huang, Y. Z. Wang, Q. Pan, Y. Wang, X. Q. Ding, K. J. Xu, Q. Wen, Anal. Chim. Acta 877 (2015) 90–99. |
| [23] | Zhao J, Lin D Q, Yao S J. Adsorption of rutin with a novel β-cyclodextrin polymer adsorbent: Thermodynamic and kinetic study. Carbohydrate Polymers, 2012, 90: 1764-1770. |
| [24] | Q. Cheng, F. Qu, N. B. Li, H. Q. Luo. Mixed hemimicelles solid-phase extraction of chlorophenols in environmental water samples with 1-hexadecyl-3-methyl-imidazolium bromide-coated Fe3O4 magnetic nanoparticles with high performance liquid chromatographic analysis. Analytica Chimica Acta 2012 715: 113–119. |
| [25] | V. Russo, R. Tesser, M. Trifuoggi, M. Giugni, M. Di Serio. A dynamic intraparticle model for fluid–solid adsorption kinetics. Computers and Chemical Engineering 2015, 74: 66–74. |
| [26] | M. R. Gonzalez-Centeno, F. Comas-Serra, A. Femenia, C. Rossello, S. Simal. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (vitis vinifera L.): Experimental kinetics and modeling. Ultrasonics Sonochemistry 2015, 22: 506–514. |
| [27] | Y. X. Zhang, Y. X. Cheng, N. N. Chen, Y. Y. Zhou, B. Y. Li, W. Gu, X. H. Shi, Y. Z. Xian. Recyclable removal of bisphenol A from aqueous solution by reduced graphene oxide-magnetic nanoparticles: Adsorption and desorption. Journal of Colloid and Interface Science 2014, 421: 85–92. |
APA Style
Bakheet, A. A. A., Zhu, X. S. (2024). Amino Acid Ionic Liquid Coated Magnetic Core Fe3O4@SiO2 Nanoparticles Coupled with UV Spectrophotometry for the Separation /Analysis Congo Red. World Journal of Applied Chemistry, 9(1), 7-14. https://doi.org/10.11648/j.wjac.20240901.12
ACS Style
Bakheet, A. A. A.; Zhu, X. S. Amino Acid Ionic Liquid Coated Magnetic Core Fe3O4@SiO2 Nanoparticles Coupled with UV Spectrophotometry for the Separation /Analysis Congo Red. World J. Appl. Chem. 2024, 9(1), 7-14. doi: 10.11648/j.wjac.20240901.12
AMA Style
Bakheet AAA, Zhu XS. Amino Acid Ionic Liquid Coated Magnetic Core Fe3O4@SiO2 Nanoparticles Coupled with UV Spectrophotometry for the Separation /Analysis Congo Red. World J Appl Chem. 2024;9(1):7-14. doi: 10.11648/j.wjac.20240901.12
@article{10.11648/j.wjac.20240901.12,
author = {Almojtaba AbdAlkhalig Ahmed Bakheet and Xia Shi Zhu},
title = {Amino Acid Ionic Liquid Coated Magnetic Core Fe3O4@SiO2 Nanoparticles Coupled with UV Spectrophotometry for the Separation /Analysis Congo Red
},
journal = {World Journal of Applied Chemistry},
volume = {9},
number = {1},
pages = {7-14},
doi = {10.11648/j.wjac.20240901.12},
url = {https://doi.org/10.11648/j.wjac.20240901.12},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.wjac.20240901.12},
abstract = {Congo red has strong tinting strength, low price that it was used in meat dyeing. Amino acid ionic liquids (AAILs) containing amino acid cations or anions are synthesized from natural amino acids, which has the characteristics of low toxicity. Nowadays, Fe3O4@SiO2@AAIL composites has seemly not been reported for the separation/analysis of congo red. In this work, Fe3O4@SiO2@AAIL nanoparticles were used as magnetic solid phase adsorbents in combination with UV spectrophotometry for separation of congo red.
},
year = {2024}
}
TY - JOUR T1 - Amino Acid Ionic Liquid Coated Magnetic Core Fe3O4@SiO2 Nanoparticles Coupled with UV Spectrophotometry for the Separation /Analysis Congo Red AU - Almojtaba AbdAlkhalig Ahmed Bakheet AU - Xia Shi Zhu Y1 - 2024/04/29 PY - 2024 N1 - https://doi.org/10.11648/j.wjac.20240901.12 DO - 10.11648/j.wjac.20240901.12 T2 - World Journal of Applied Chemistry JF - World Journal of Applied Chemistry JO - World Journal of Applied Chemistry SP - 7 EP - 14 PB - Science Publishing Group SN - 2637-5982 UR - https://doi.org/10.11648/j.wjac.20240901.12 AB - Congo red has strong tinting strength, low price that it was used in meat dyeing. Amino acid ionic liquids (AAILs) containing amino acid cations or anions are synthesized from natural amino acids, which has the characteristics of low toxicity. Nowadays, Fe3O4@SiO2@AAIL composites has seemly not been reported for the separation/analysis of congo red. In this work, Fe3O4@SiO2@AAIL nanoparticles were used as magnetic solid phase adsorbents in combination with UV spectrophotometry for separation of congo red. VL - 9 IS - 1 ER -